Sodium-ion vs. lithium-iron-phosphate batteries
Researchers from the Technical University of Munich (TUM) and RWTH Aachen University in Germany have compared the electrical performance of high-energy sodium-ion batteries (SIBs) to that of a state-of-the-art high-energy lithium-ion battery (LIBs) with a lithium-iron-phosphate (LFP) cathode.
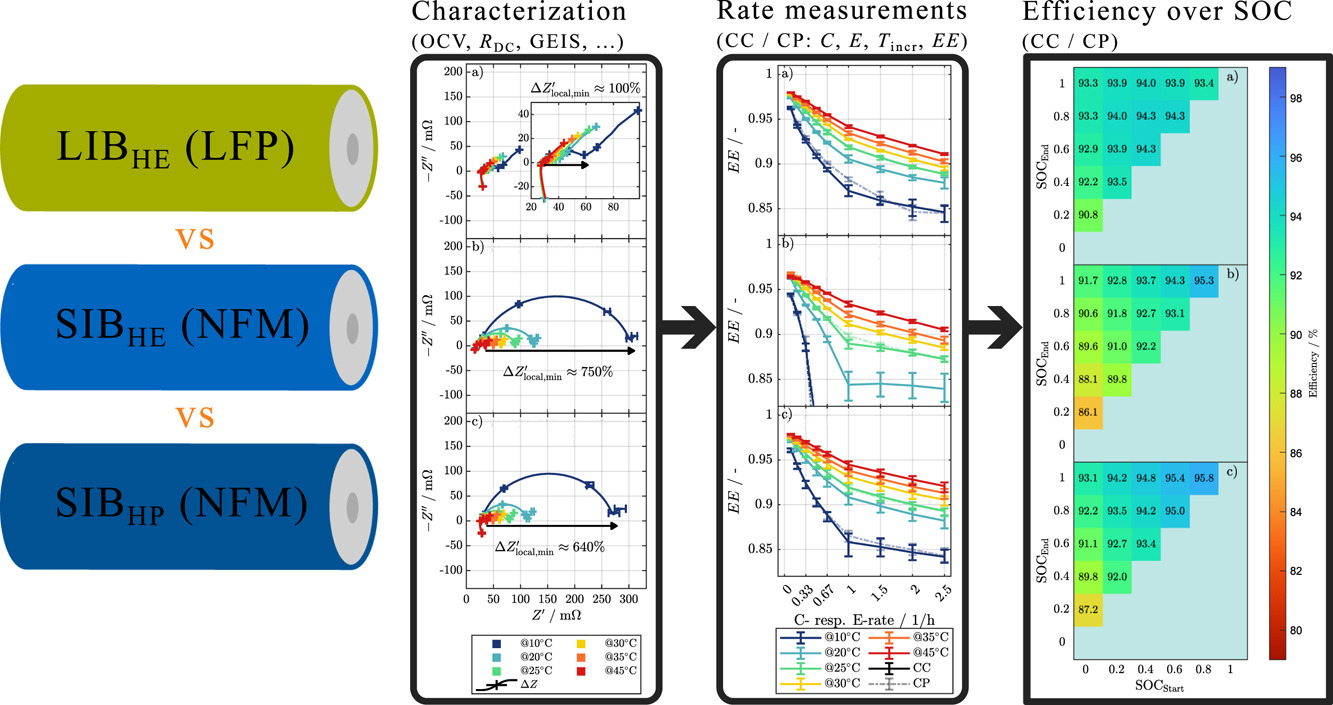
The team found that the state-of-charge and temperature have a higher influence on the pulse resistance and the impedance of the SIBs than the LIBs, which may influence design choices and suggests that SIBs may require more sophisticated temperature and charge management systems to optimize performance, especially at lower charge levels.
- To explain pulse resistance further: the term refers to how much a battery voltage drops when a sudden power demand is applied. Therefore, the research indicates that sodium-ion batteries are more affected by charge level and temperature than lithium-ion batteries.
Research:
“Sodium-ion batteries [SIBs] are generally seen as a drop-in replacement for LIBs,” the scientists stated. “Nevertheless, the differences in the electrochemical behavior of sodium and lithium require adaptions on both the anode and the cathode. While for lithium-ion batteries [LIBs] usually graphite is used as anode material, for SIBs hard carbon is currently seen as the most promising material for SIBs.”
They also explained that their work was intended to fill a gap in the research, as there is still a lack of knowledge about the electrical behavior of SIBs in terms of varying temperatures and state-of-charges (SOCs).
The research team conducted, in particular, electrical performance measurements at temperatures ranging from 10 degrees C to 45 degrees C and open-circuit voltage measurements of the full-cell at different temperatures as well as half-cell measurements of the corresponding cells at 25 C.
“Furthermore, we investigated the influence of temperature and SOC on both direct current resistance (R DC) and galvanostatic electrochemical impedance spectroscopy (GEIS),” it specified. “To examine the useable capacity, useable energy, and energy efficiency under dynamic conditions, we performed rate capability tests by applying different load rates at different temperatures.”
The researchers measured a lithium-ion battery, a sodium-ion battery with a nickel-manganese-iron cathode, and a lithium-ion battery with an LFP cathode. All three showed voltage hysteresis, meaning their open-circuit voltage differed between charging and discharging.
“Interestingly, for SIBs, the hysteresis is primarily occurring at low SOCs, which is, according to half-cell measurements, likely due to the hard carbon anode,” the academics emphasized. “The R DC and impedance of the LIB show very little dependence on the SOC. In contrast, for SIBs, the R DC and impedance significantly increase at SOCs below 30%, while higher SOCs have the opposite effect and lead to lower R DC and impedance values.”
Moreover, they ascertained that the temperature dependence of R_DC and impedance is higher for SIBs than LIBs. “The LIB tests do not show a significant influence of the SOC on the round-trip efficiency. In contrast, cycling the SIBs from 50% to 100% SOC can reduce efficiency losses by more than half compared to cycling from 0% to 50%,” they further explained, noting that the efficiency of SIBs grows drastically when cycling the cells in a higher SOC range compared to a lower SOC range.
Post time: Feb-18-2025